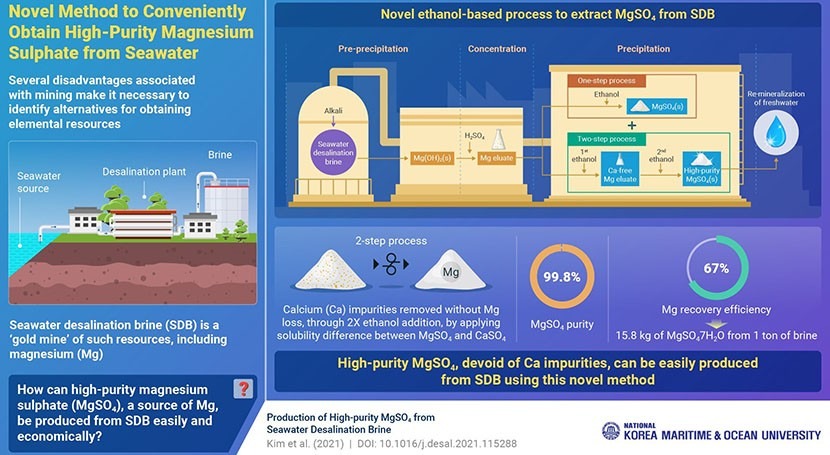
Given that mining to extract high-grade mineral ores is wastefully energy intensive, exhaustible, and bad for the environment, scientists have been scouting for alternatives. A group of Korean researchers, led by Professor Myoung-Jin Kim, from Korea Maritime and Ocean University, have now succeeded in extracting high-quality minerals from just seawater. Specifically, they have achieved the extraction of 99.8% pure magnesium sulfate (MgSO4), from seawater desalination brine (SDB).
Speaking about the motivation behind the study, Prof. Kim explains that "since we have already developed a sophisticated seawater desalination process to address the world's water needs, why not couple it with the beneficial process of mineral extraction! In this way, we believe that such extraction can be performed in an energy-efficient, sustainable, and environment-friendly manner." The team's findings have been published online, as a research article, on 15th December 2021, in the journal Desalination.
The researchers have achieved the extraction of 99.8% pure magnesium sulfate (MgSO4), from seawater desalination brine (SDB)
Further, the team has not only coupled the two processes, but has also developed a novel and subtle ethanol-based process to extract MgSO4. Initially, the researchers followed steps such as alkali-based magnesium hydroxide pre-precipitation from brine, and sulfuric acid-based magnesium concentration. Finally, they treated the magnesium eluate, twice, with ethanol—the first time, to remove calcium impurities, and the second time, to precipitate the high-purity MgSO4. Interestingly, this final two-step process used the difference in solubility between magnesium and calcium sulfates in ethanol, to achieve up to 67% magnesium recovery efficiency.
Owing to the cost-effectiveness of mineral extraction from SDB, the researchers state that the obtained MgSO4 may not only be used for re-mineralizing desalinated freshwater, but also find potential applications in the pharmaceutical and food industry. Prof. Kim adds that they "hope that our study encourages further research on alternative mineral extraction processes."
Indeed, humanity, with the aid of such science, can hope to reverse environmental damage, while sustainably meeting its needs for continued technological advancements.