What is the formula of water?

Water is the most abundant molecule in living beings: it makes up between 70 and 90% of the weight of most organisms.
Index
1 . H2O
Water is a substance formed by two hydrogen atoms and one oxygen atom, so its chemical formula is H2O. The chemical bonds between the oxygen atom and the hydrogen atoms form a 104.5º angle.
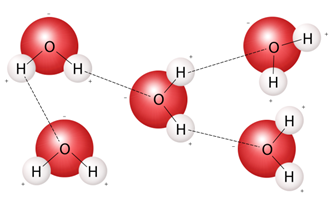
Hydrogen bonds seen as dotted lines (Source: USGS)
In addition, water molecules can form hydrogen bonds with neighbouring molecules. At room temperature each water molecule forms on average approximately 3 hydrogen bonds. This is the reason why water is a liquid at room temperature.
2 . A polar molecule
The water molecule has a neutral charge but, since it is formed by a very electronegative element, oxygen, and a very electropositive element, hydrogen, it is a polar molecule. This means it behaves as if one side of the molecule had a negative charge, and the other side, a positive charge.
This uniqueness has implications for water's properties; for example, it can act as a solvent for many substances, and it remains liquid under a broad range of temperatures





