The rapid increase in global industrialization in the past centuries has resulted in the disposal of excessive toxic pollutants into the environment, seriously threatening the aquatic ecosystem and human health. Peroxymonosulfate-based advanced oxidation processes (PMS-AOPs) are appealing techniques to treat these toxic pollutants.
Leveraging diverse reactive oxygen species (ROS), AOPs are sought to oxidize or even mineralize recalcitrant and toxic organic pollutants. Single-atom catalysts (SACs) are featured with maximized atom utilization as well as uniform and well-defined active sites, holding great promise for effective and selective PMS activation. However, the structure-activity/selectivity relationships have not yet been well revealed owing to multiple ROS generation pathways and their complex interplay.
Recently, a research team led by Prof. Shaobin Wang from The University of Adelaide, Australia, and Prof. Hui Zhang from Wuhan University, China, has elucidated the mechanisms of PMS activation by single-atom iron catalysts and identified the relationships of the geometric and electronic structures of single atom Fe centers to selective production of different reactive species/pathways. The results were published in Chinese Journal of Catalysis.
Single-atom catalysts revolutionize water purification: efficient and selective generation of reactive oxygen species for global water challenges. Credit: Chinese Journal of Catalysis
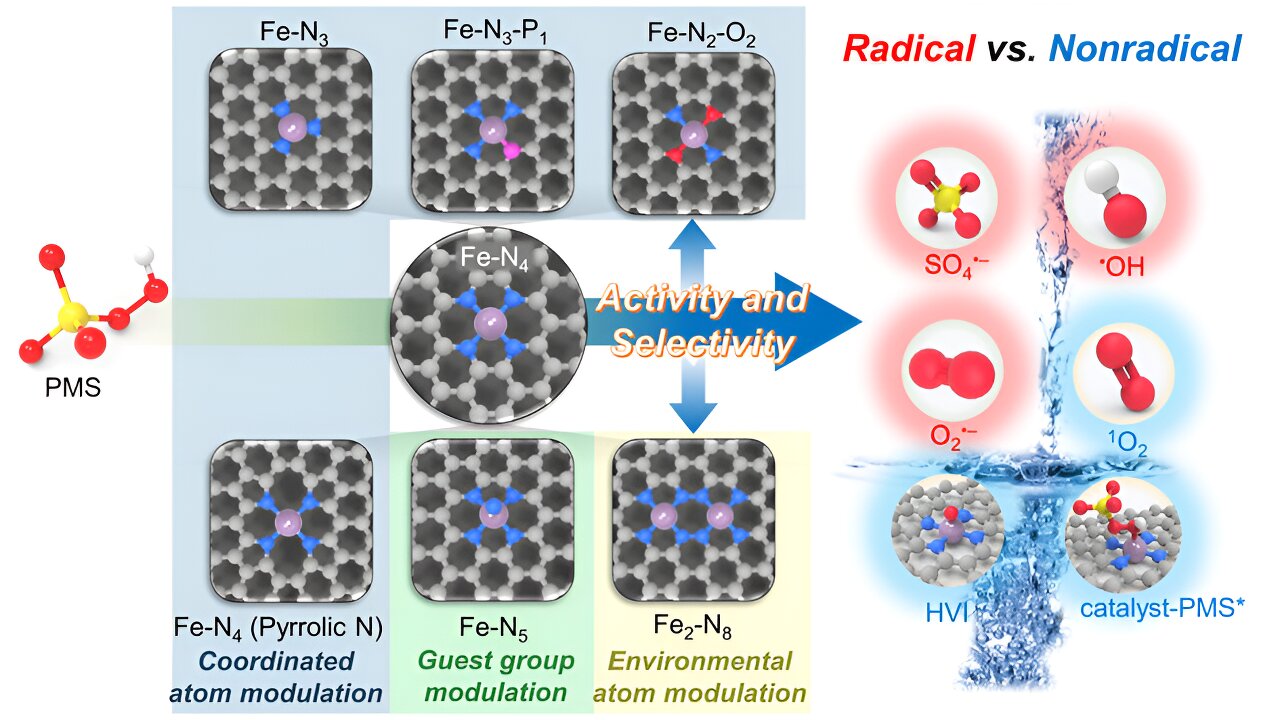
The activation of PMS by single-atom sites usually proceeds via three steps. The first step is the adsorption of HSO5– onto the single atom Fe center with varying molecular alignments. The second step is the charge transfer between the active Fe center and adsorbed HSO5–, resulting in the change of charge density and even cleavage of O–O bond of HSO5–. The third step is the detachment of the activated HSO5– through a spontaneous release of the reactive species (such as free radicals and 1O2) or decomposition of the surface-bound species with other substrates (such as catalyst-PMS* and FeIV=O) to regenerate the active site.
Due to the complexity of PMS activation with multiple steps and ROS generation with multiple reaction intermediates, diverse ROS are produced even at a singular atomic Fe site. While theoretical tools can efficiently determine the energetically optimal PMS adsorption structure, the ultimate generation of specific ROS necessitates moderate reaction-free energy in each reaction step. Therefore, the control of ROS selectivity requires managing both PMS adsorption energy and the reaction-free energy of crucial elementary steps.
In this context, regulating the electronic structure of the active site is an effective way to govern PMS activation activity and ROS selectivity for establishing the relationships. This involves a moderate increase of the charge density of Fe sites by constructing Fe–N2–O2 and Fe–N5 sites to enhance 1O2 and FeIV=O generation, as well as an Fe–N3 site for catalyst-PMS* complex generation. Further elevation of the charge density by Fe–N3–P1 and Fe–N4 (pyrrolic N) sites favors radical generation.